![]()

|
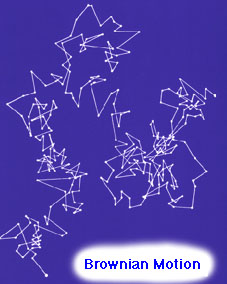
Einstein on Brownian Motion ª@ ª@ ª@ ª@ ª@ ª@ Original picture in Perrin's Atoms |
 |
Einstein on Brownian Motion
It is well known that Einstein published in 1905 five important papers, including two papers on the special relativity. Another is the paper on photo-electric effect for which he received the Nobel Prize later. And still another is the paper on the so-called Brownian Motion which encouraged Jean Perrin to pursue his experimental work for confirming the kinetic theory and showing the existence of atoms. Here is the beginning paragraph of this famous paper.
In this paper it will be shown that, according to the molecular-kinetic theory of heat, bodies of a microscopically visible size suspended in liquids must, as a result of thermal molecular motions, perform motions of such magnitudes that they can be easily observed with a microscope. It is possible that the motions to be discussed here are identical with so-called Brownian molecular motion; however, the data available to me on the latter are so imprecise that I could not form a judgment on the question. (John Stachel, ed., Einstein's Miraculous Year: Five papers that changed the face of physics, Princeton University Press, 1998, 85; Einstein's original papers are included in the Collected Papers of Albert Einstein, vol. 2)
What is crucial in this paper is that Einstein predicted "motions of such magnitudes that they can be easily observed with a microscope". Many people, before Einstein, studied and performed experiments on, the Brownian motion, but they could not obtain any decisive results. The reader should read Stachel's introduction for details. In order to supplement Mayo's description, I will quote Stachel's nice summary on the significance of Einstein's paper.
In summary, the study of previous explanations of Brownian motion shows that three elements of Einstein's approach are characteristic of his decisive progress: (1) he based his analysis on the osmotic pressure rather than on the equipartition theorem; (2) he identified the mean-square displacements of suspended particles rather than their velocities as suitable observable quantities; and (3) he simultaneously applied the molecular theory of heat and the macroscopic theory of dissipation to the same phenomenon, rather than resticting each of these conceptual tools to a single scale, molecular or macroscopic. (op. cit., 77)
The second element (2), in particular, is concerned with the effects observable with a microscope. It should be instructive to see what Perrin says on this point.
Einstein and Smoluchowski have defined the activity of the Brownian movement in the same way. Previously we had been obliged to determine the "mean velocity of agitation" by following as nearly as possible the path of a grain. Values so obtained were always a few microns per second for grains of the order of a micron.
But such evaluations of the activity are absolutely wrong. The trajectories are confused and complicated so often and so rapidly that it is impossible to follow them; ... Similarly, the apparent mean speed of a grain during a given time varies in the wildest way in magnitude and direction, and does not tend to a limit as the time taken for an observation dcreases, ...
Neglecting, therefore, the true velocity, which cannot be measured, and disregarding the extremely intricate path followed by a grain during a given time, Einstein and Smoluchowski chose, as the magnitude characteristic of the agitation, the rectilinear segment joining the starting and end points; in the mean, this line will clearly be longer the more active the agitation. The segment wil be the displacement of the grain in the time considered. Its projection on to a horizontal plane, as perceived directly in the microscope under ordinary conditions (microscope vertical), will be its horizontal displacement. (Perrin, Atoms, 109-110)
Thus, by neglecting the intricate path of a grain (see the figure on the top) and by noticing another quantity, far more easily extracted from observation, Einstein and Smoluchowski opened a new promising way. Perrin, following Einstein's line of reasoning, continues as follows:
In accordance with the conclusions arrived at from qualitative observation, we shall regard the Brownian movement as completely irregular in all directions at right angles to the vertical. This is scarecely a hypothesis; moreover, we shall verify all its consequences.
This being granted, and without any further hypothesis whatever, it can be proved that the mean displacement of a grain is doubled when the time is increased fourfold; it becomes tenfold when the time is increased a hundredfold and so on. More precisely, it is proved that the mean square ee of the horizontal displacement during the time t increases in proportion to that time.
The same result holds for half this square or the mean square xx of the projection of the horizontal displacement along an arbitrary horizontal axis. In other words, for a given kind of grain (in a given fluid) the quotient xx/t is constant. ... this quotient characterizes the activity of the Brownian movement for any particular grain. (110-111)
We will not pursue Einstein's theory any further, since the point of Mayo's argument is, "How can we establish this claim of complete irregularity of the Brownian motion?" Here, the role of statistical test is crucial. (Continued to Bull's Eye)
Last modified Jan. 27, 2003. (c) Soshichi Uchii