

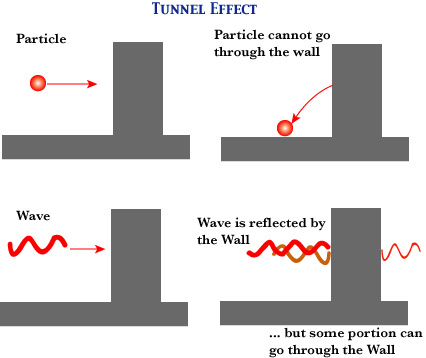
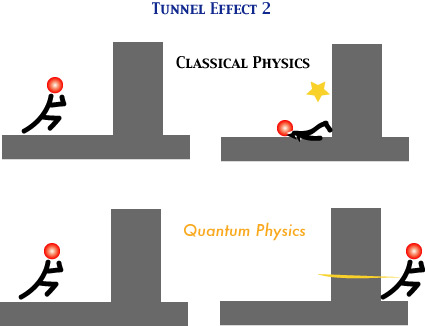
Tunnel Effect
De Broglie's idea of "matter as wave" suggests a startling consequence, and that's what is called the tunnel effect. More precisely, Schroedinger's wave equation can show this rigorously. Unlike the classical mechanics of particles, quantum mechanics allows light as well as particles (such as electrons and protons) to appear even where the "wall" of potential should prevent them from appearing. In the following figure, you may imagine the wall as the "wall of potential", in that any particle must have an energy, greater than a certain amount, for going through it and appearing on the other side. But even when the particle has a lower energy than that, it can go through the wall, just as a wave can appear on the other side (since its oscillation can go through the wall). Since particles as well as light have particle-wave duality, matter (with an appropriate energy) can go through the wall according to quantum mechanics. This can explain the spontaneous disintegration of radioactive substances (such as radium); even though the strong interaction within the nucleus forms a high wall of potential, alpha-distintegration can occur because of the tunnel effect.
Bohr briefly mentions this (58); but for a more rigorous treatment, the reder is referred to Dr. Tomonaga's book (1997, 101-106).
Bohr, N. (1958) Essays 1933-1957 On Atomic Physics and Human Knowledge
Tomonaga, S. (1997) 量子力学 II(第2版)、みすず書房。
See also a useful site on Microphysics, at Kyushu University: http://www2.kutl.kyushu-u.ac.jp/seminar/MicroWorld/MicroWorld.html