

Bohr's Quantum Model of the Hydrogen
Now Niels Bohr comes on the scene. Facing the difficultites of the Rutherford Model (which nevertheless has a great virtue of being based on experimental facts), Bohr tried to remedy it by introducing the idea of quantum. In a sense, he tried to apply Einstein's hypothesis of light quantum to the atomic structurte. In Bohr's own words (the 1st paper in the text, written in 1925), he introduced the following postulates:
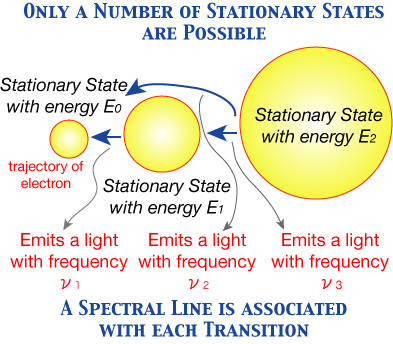
(1) An atomic system possesses a certain manifold of states, the "stationary states", to which corresponds in general a discrete sequence of energy values and which have a peculiar stability. This latter shows itself in that every change in the energy of the atom must be due to a "transition" of the atom from one stationary state to another.
(2) The possibility of emission and absorption of radiation by the atom is conditioned by the possibility of energy changes of the atom, in such a way that the frequency of the radiation is connected with the energy difference between the initial and final states by the formal relation
hν= E1 - E2 .
It should be noted that in Balmer's Formula (rewritten in the manner of Rydberg), the same form of the difference between two terms appeared. Consequently, Balmer's Formula can be easily derived from these two postulates. And the frequency ν of a spectral line (of the hydrogen atom) can be calculated according to this relation.
However, Bohr (in his 1925 paper) did not mention another assumption for his calculation of the size of the hydrogen atom (roughly, the diameter of the trajectory of electron, assuming that the trajectory is circular) in a stationary state; this was clearly stated in his 1913 paper. That is,
(3) In a stationary state, the atomic system can be treated according to the classical mechanics (the force between the nucleus and the electron acts according to the inverse square law).
Bohr's idea that an atom has many different stationary states was later supported by experimental data, such as Franck-Herz experiment (1914). Also, his idea opened a way for extending the notion of "quantization" (stated in the preceding (1) and (2)) to other phenomena; the reader is referred to Dr. Tomonaga's book.
Bohr, N. (1913), "On the Constitution of Atoms and Molecules, Part I", Philosophical Magazine 26, 1913. 邦訳は、物理学古典論文叢書10『原子構造論』(東海大学出版会、1969)に収録。
Bohr, N. (1934), Atomic Theory and the Description of Nature, Ox Bow Press (reprint)
Tomonaga, S. (1969) 量子力学 I、みすず書房。
See also a useful site on Microphysics, at Kyushu University: http://www2.kutl.kyushu-u.ac.jp/seminar/MicroWorld/MicroWorld.html