

Researches on the Structure of Atoms
Let us see some of the major discoveries which prepared the ground for the quantum mechanics. Bohr's description may be a bit hard for us without this knowledge, at least in terms of intuitive images. Again, for a more detailed explanation, the reader is referred to Dr. Tomonaga's textbook.
Balmer's Formula
The elements are known to emit a characteristic spectrum under suitable conditions; it has a series of lines and these can be used for identifying the substance (element) in question; thus one can easily suspect that these lines may be a clue for knowing the hidden (atomic) structure of the elements. In 1885, a Swiss school teacher J. J. Balmer proposed a formula (see, e.g., Dr. Tomonaga's book) describing the regularity of the lines in the emission spectrum of hydrogen, the simplest element. The lines Balmer considered for proposing his formula look like this:
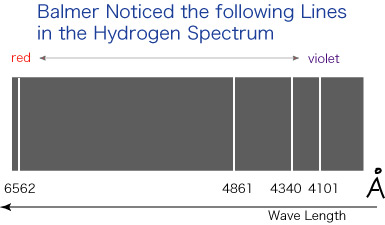
More works in this field were done by other people, including J. R. Rydberg, and Rydberg found that frequency, rather than wavelength, is to be used for expressing such regularities (around 1890).
Zeeman Effect
Another phenomenon attracted physicists' attention is the Zeeman effect. A Dutch physicist P. Zeeman discovered (1897) a strange effect on the emission spectrum by placing an element within a magnetic field. A line in the emission spectrum of an element, splits into three different lines (one on both sides of the original line) if placed in a magnetic field, and the displacement is proportional to the strength of the magnetic field.
Although it was possible to explain this effect even within classical electromagnetism, anomalous Zeeman effect shows more spectral lines than three, and it is harder to explain. It is due to interactions between trajectories and spin.
Rutherford Model of the Atomic Structure
Ernest Rutherford made an epoch in nuclear physics. For instance, he showed that an element can be changed into another by bombardment of alpha particles. But we are here concerned with his research on the atomic structure (1911). By his careful study of scattering of alpha particles around an element, he concluded that an atom must have a structure quite analogous to a planetary system---a heavy nucleus at the center and electrons going around this nucleus.
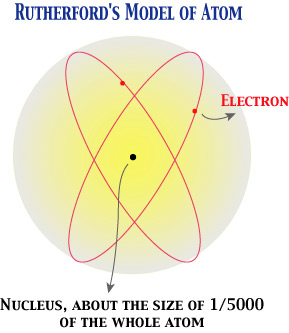
Although this model still had grave difficulties (resolved only after the quantum mechanics emerged), Rutherford's reasoning leading to this model from experimental findings is still quite worthwhile. He treated scattering of alpha particles in terms of classical physics and statistical reasoning. Basically, he reasoned that the actual phenomena of scattering would be quite improbable unless we posit the preceding model of atomic structure.
Here is the crucial part of Rutherford's reasoning. The concept of "cross section" recurs again and again in nuclear physics, as is well known with the development of the atomic bomb. Suppose a substance is bombard by a beam (with even density) of alpha particles. Then we can observe what is called "scattering of alpha particles" around a nucleus, and this scattering can be calculated (on Rutherford model) as follows (equations are in Dr. Tomonaga's book):
In general, the deflection angle is greater the smaller the impact parameter (for instance, the particle coming toward the center of the nucleus will be deflected 180 degrees, and some particles a bit away from the nucleus will be deflected 90 degrees).
By comparing the cross section calculated on the preceding model (and classical electromagnetic theory) with the data obtained by experiment, Rutherford came to his conclusion. However, his model could not explain the size of a whole atom (with such a structure), nor the lines in the emission spectrum, nor the stability of an element. Young Bohr, studying with Rutherford, were to challenge these difficulties.
Tomonaga, S. (1969) 量子力学 I、みすず書房。
See also a useful site on Microphysics, at Kyushu University: http://www2.kutl.kyushu-u.ac.jp/seminar/MicroWorld/MicroWorld.html